

Stilla Technologies Digital PCR System
naica®Crystal Digital PCR System
NaicaTMCrystal Digital PCR System
Azure Biosystems Real-Time PCR Systems

When it comes to quantitative fluorescent Western blotting, more is definitely not always better. Reliable quantitative Western blot data can only be attained by loading the proper amount of sample. Loading too much protein can result in signal saturation or self-quenching of the fluorophore that is used for detection.
So how do you know how much lysate to load? First, start off by measuring the concentration of your lysate samples with a protein assay such as BCA or Bradford. Once you know the concentration of your samples you can then ensure that you are loading the same amount of each.
Unfortunately, we don’t live in a perfect world, so you will still need a means of compensating for gel loading mishaps and transfer inefficiencies. The emerging consensus on the best way to normalize Western blots is to normalize to total protein. Many journals have embraced total protein normalization (TPN) and some, such as the Journal of Biological Chemistry, even require authors to use TPN when publishing quantitative data from Western blots or else to validate the use of their housekeeping protein1, 2.
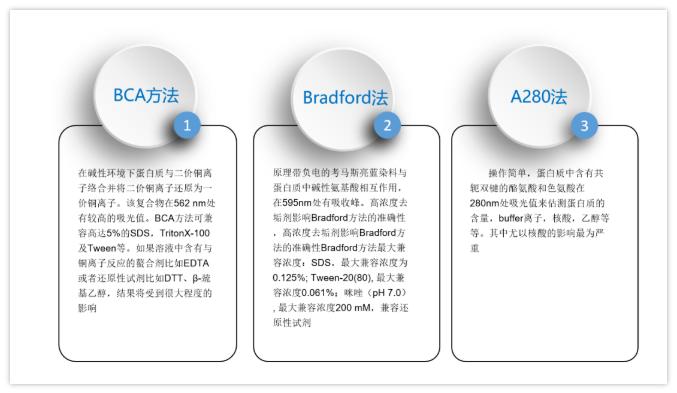
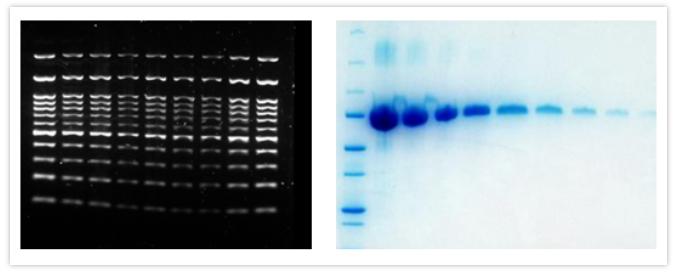
In the experiment below, a broad range of HeLa lysate from 0.2-50µg was loaded to examine the performance of the target protein, STAT3 in comparison to a loading control, GAPDH and total protein membrane staining. Although Total-PVDF membrane stain is linear up to 50µg of lysate, this is clearly not the ideal amount of lysate to load for STAT3, the target protein, since the signal is no longer linear above 12.5µg. Normalization with the loading control GAPDH poses a serious issue since it is only linear below 1µg of lysate, making its use very limited.
Generally speaking, we do not advise loading more than 20µg of lysate as it will often cause problems with protein quantitation. For the best quantitative fluorescent Western blots, don’t forget to apply an appropriate normalization strategy and load the right amount of protein.
Azure Imager system

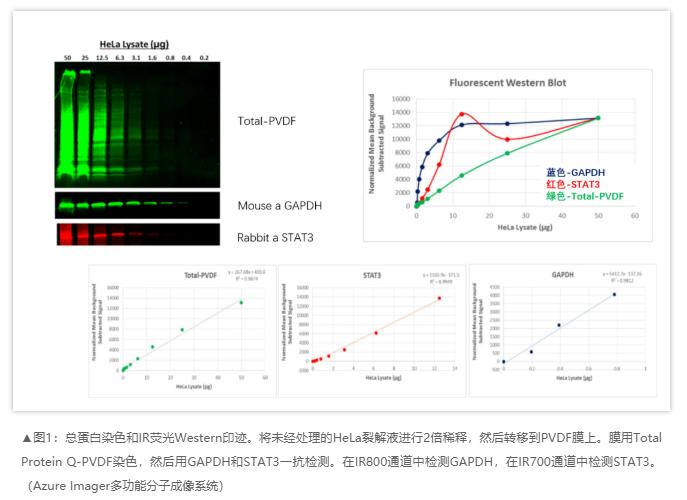
☑ Dual laser near infrared imaging: high intensity, high signal-to-noise ratio, and low crosstalk.
☑ RGB visible fluorescence multifunctional detection: mouse imaging, genetically modified plant screening, model organism zebrafish imaging, petri dish imaging, etc.

☑ Simultaneous 4-channel fluorescence imaging makes detection more efficient.

☑ Highly sensitive chemiluminescence and color Marker imaging.
☑ Color protein gel and nucleic acid gel imaging.
References:
1. Collecting and presenting data. The Journal of Biological Chemistry.
website.:http://jbcresources.asbmb.org/collecting-and-presenting-data#blot. Accessed February 4, 2019.
2. Fosang AJ and Colbran RJ. Transparency Is the Key to Quality. J Biol Chem. Dec. 11 2015. 290(50). 29692–29694. PMCID: PMC4705984.
